FDA Approves Preservative-Free XELPROSTM
XelprosTM (latanoprost ophthalmic emulsion 0.005%) gains FDA approval for reducing elevated intraocular pressure (IOP) in patients with open-angle glaucoma and ocular hypertension, Sun Pharma (India’s top specialty generic pharmaceutical) and SPARC (a clinical stage bio-pharmaceutical company) announced in a joint press release.
About XELPROSTM
XELPROSTM is an off-white to pale yellow, translucent emulsion of latanoprost 0.005% (50 mcg/mL), a prostaglandin analogue that is used as a treatment for open-angle glaucoma or ocular hypertension.
The recommended dosage of XELPROSTM is one drop in the affected eye(s) once daily in the evening. The reduction of IOP commences approximately 3 to 4 hours after administration and the maximum effect is reached after 8 to 12 hours that lasts for at least 24 hours.
Preservative-Free Latanoprost
XELPROSTM is the only FDA-approved BAK-free version of Latanoprost. It joins three other FDA-approved topical glaucoma medications completely free of preservatives: Zioptan (tafluprost ophthalmic solution 0.0015%), Cosopt PF (dorzolamide-timolol ophthalmic solution 2%/0.5%), and Timoptic in Ocudose (timolol maleate ophthalmic solution 0.25% and 0.5%). Because they are preservative free, these medications are known to be better tolerated than their BAK-preserved counterparts, which in effect, results to better adherence to treatment.
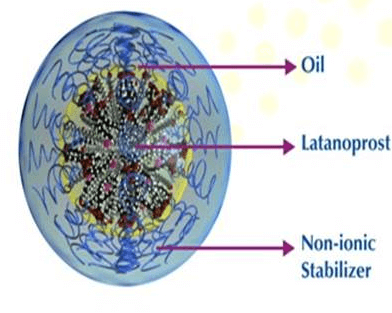
Developed with SPARC’S Swollen Micelle Microemulsion Technology
XELPROSTM is developed using SPARC’s proprietary Swollen Micelle Microemulsion (SMM) technology, which helps to solubilize ophthalmic drugs that have limited water solubility or no solubility thus eliminating the need for quaternary ammonium preservative/ surfactant benzalkonium chloride (BAK), which may be damaging to the eye.
Safety and Efficacy
Across randomized clinical trials of patients with open-angle glaucoma or ocular hypertension with a mean baseline Intraocular pressure (IOP) of 23-26 mmHg, XELPROSTM administered once daily in the evening lowered IOP by a mean of up to 6-8 mmHg.
The most frequently reported adverse reactions were eye pain/stinging upon instillation and ocular hyperemia (redness), reported in 55% and 41% of patients treated with XELPROSTM respectively. Less than 1% of patients discontinued therapy because of intolerance to these adverse events.
Aside from the frequently reported adverse reactions, common side effects of all Topical Latanoprost ophthalmic products are:
- Pigmentation of the iris (likely to be permanent), periorbital tissue (eyelid) and eyelashes can occur; and
- Eyelash changes such as gradual change to eyelashes including increased length, thickness and number of lashes, which is usually reversible upon discotinuation of treatment.
XELPROSTM will be commercialized in the U.S. by Sun Ophthalmics.
SOURCE: myxelpros.com
CONTRAINDICATIONS
XELPROS is contraindicated in patients with a known hypersensitivity to latanoprost, or any other ingredients in this product.
WARNINGS AND PRECAUTIONS
Pigmentation: XELPROS may cause changes to pigmented tissues. The most frequently reported changes are increased pigmentation of the iris, periorbital tissue (eyelid), and eyelashes. Pigmentation is expected to increase as long as XELPROS is administered. After discontinuation of XELPROS, iris pigmentation is likely to be permanent. Patients who receive treatment should be informed of the possibility of increased pigmentation. The long-term effects of increased pigmentation are not known.
Eyelash Changes: XELPROS may gradually change eyelashes and vellus hair in the treated eye, including increased length, thickness, pigmentation, and number of lashes. The changes are usually reversible upon discontinuation of treatment.
Intraocular Inflammation: XELPROS should be used with caution in patients with a history of intraocular inflammation (iritis/uveitis) and should generally not be used in patients with active intraocular inflammation.
Macular Edema: XELPROS should be used with caution in aphakic patients, in pseudophakic patients with a torn posterior lens capsule, or in patients with known risk factors for macular edema.
Herpetic Keratitis: XELPROS should be used with caution in patients with a history of herpetic keratitis. XELPROS should be avoided in cases of active herpes simplex keratitis because inflammation may be exacerbated.
Bacterial Keratitis: There have been reports of bacterial keratitis associated with the use of multiple-dose containers of topical ophthalmic products.
Use with Contact Lenses: Contact lenses should be removed prior to administration of XELPROS and may be reinserted 15 minutes following administration.
ADVERSE REACTIONS
The most common ocular adverse reactions (incidence ≥5%) for XELPROS were eye pain/stinging, ocular hyperemia, conjunctival hyperemia, eye discharge, growth of eyelashes, and eyelash thickening.
DRUG INTERACTIONS
Precipitation may occur if drugs containing thimerosal are used concomitantly with XELPROS. If such drugs are used, they should be administered at least 5 minutes apart.
Please see the Full Prescribing Information.
References
- Sun Pharma and SPARC’s XELPROS gets USFDA nod [Internet]. The New Indian Express. 2018 [cited 2018 Sep 17]. Available from: http://www.newindianexpress.com/business/2018/sep/15/sun-pharma-and-sparcs-xelpros-gets-usfda-nod-1872327.html
- Sun Pharma and SPARC Announce US FDA Approval of XELPROS™ to Treat Open-angle Glaucoma or Ocular Hypertension [Internet]. Mumbai, India, Princeton, NJ,; 2018 [cited 2018 Sep 17]. p. 6. Available from: https://myxelpros.com/pdf/xelpros-news-release.pdf
- Swollen Micelle MicroemulsionTM (SMM) [Internet]. Sparc.life. 2018 [cited 2018 Sep 17]. Available from: https://www.sparc.life/research-programs/xelpros
Related Articles:
- PolyActiva Commences Clinical Trial of Biodegradable Ocular Implant for Glaucoma
- VYZULTA™: A New FDA Approved Glaucoma Medication with Dual Action
- Sustained Drug Delivery for Glaucoma Treatment – An Update from the American Glaucoma Society (AGS) 2017 Annual Meeting
- Simple Drops™ – Glaucoma Treatment Eye Drops
- Vesneo: A New Glaucoma Medication With Dual Action
Don’t delay getting checked for glaucoma.
Make an appointment with an eye doctor in your area now. If you live in the greater Los Angeles area and would like Dr. Richardson to evaluate your eyes for glaucoma call 626-289-7856 now. No referral required. Appointments are available, Tuesday through Saturday.