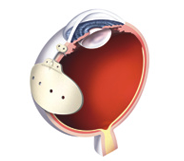
Given that glaucoma drainage devices have been around for almost fifty years, there has been surprisingly little innovation. All modern implants have two components: a tube that is placed inside the eye; and a plate onto which the fluid drains. Some glaucoma drainage devices also have a valve that regulates flow out of the eye. Other than the presence or absence of a valve, the main difference among implants are size and material used.
Types of Glaucoma Drainage Devices
Non-Valved Glaucoma Drainage Devices
Molteno™
The original glaucoma drainage device is made of polypropylene. Still being used by glaucoma surgeons today, it is now available with either a single plate (130mm2) or double plate (270mm2) reservoir. The additional plate adds surface area which is believed to further lower the intraocular pressure (IOP).
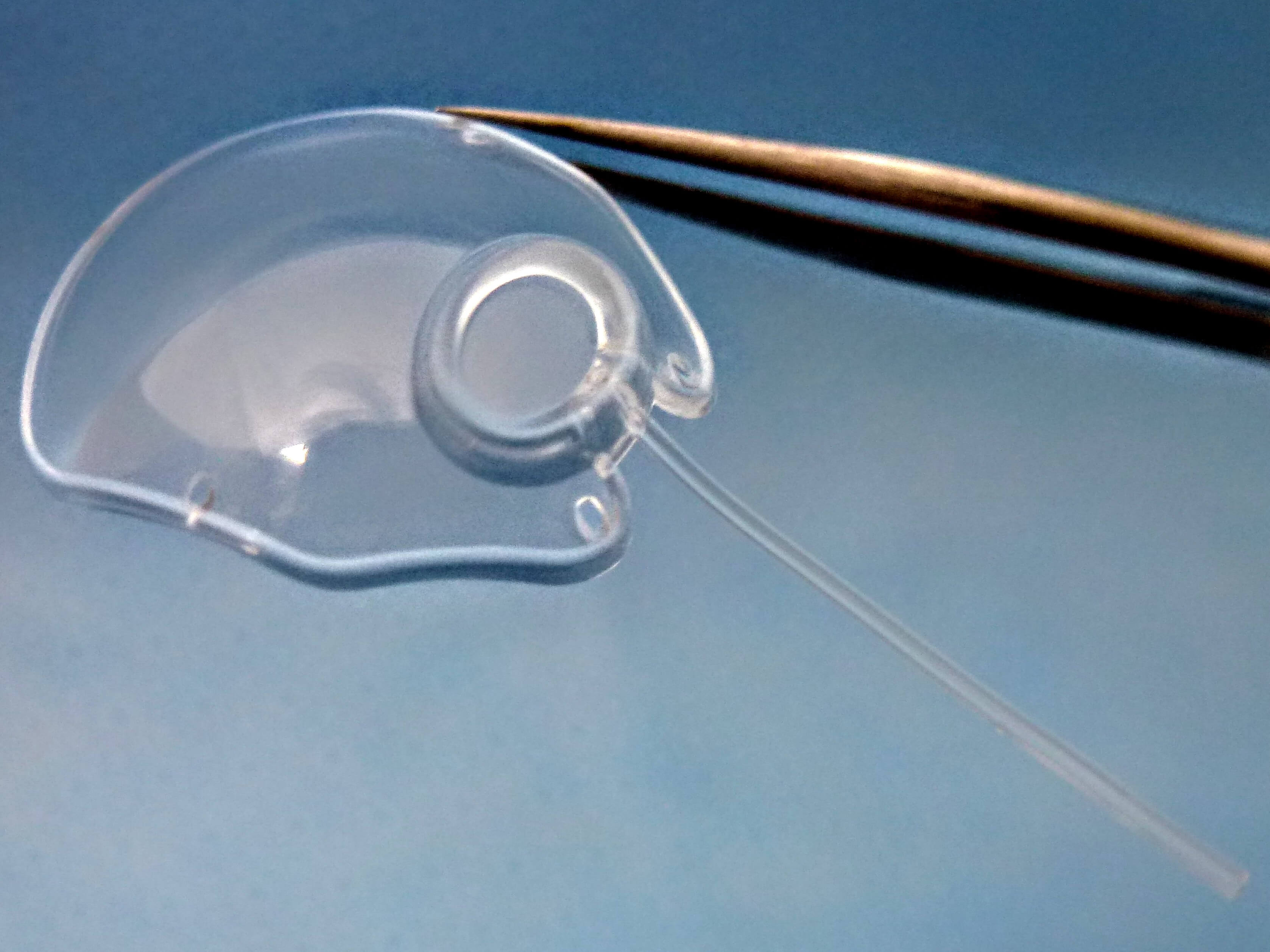
[Molteno3 S-Series. Photo credit: Molteno Ophthalmic Ltd
OCTOBER 2018 UPDATE:
The Molteno Single Plate Glaucoma Implant and the Molteno Double Plate Implants are still in use today, however, they have been superseded by the Molteno3 Glaucoma Drainage Devices. The original Molteno implants are no longer sold in the USA. Three implants are available for surgeons in the USA: the two, latest design, Molteno3 S-Series implants (SS-185mm2 and SL-245mm2) and the tiny, older style Molteno paediatric/microphthalmic Implant, (for rare cases of glaucoma in children with microphthalmic eyes. Most children with glaucoma have large eyes and use the same Molteno3 implants as adults).
There is no question that IOP was lowered further when a Double Plate implant was used. There is a good body of evidence to support the claim.
Update on Molteno/Molteno3 design changes:
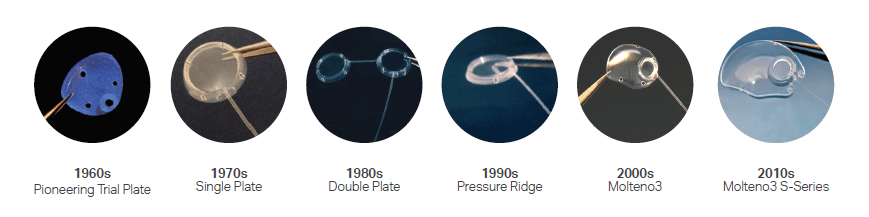
[Updates and photos provided by MOLTENO Ophthalmic Ltd]
Baerveldt®
This barium-impregnated silicon implant was introduced in 1992 by Dr. George Baerveldt. Unlike the Unlike the original Molteno™ implant which is round, the Baerveldt® implant has a wing-like appearance. These “wings” fit underneath the eye muscles allowing for a larger surface area onto which aqueous fluid can flow. In fact, the Baerveldt® implants are the largest available, ranging from 250mm2 to 425mm2
Valved Glaucoma Drainage Devices
Valved implants were created to restrict the flow of fluid out of the eye. Why would you want to do that? Because without some resistance to flow the aqueous humor would simply flood the subconjunctival space resulting in an IOP of zero. Eventually the body creates a capsule of scar tissue around the implant which acts to limit flow. Until that happens, however, something must be done to restrict the amount of fluid leaving the eye.
Surgeons will often tie the tubes of non-valved implants. These ties will either dissolve over time or have to be removed later. If the knot is undone too soon then a sudden drop in IOP will occur. Until the tube is allowed to open, however, the IOP will remain high and there will be little effect from the surgery.
Tying off the tube is not necessary when a valve is present. By design a valve should remain closed below a threshold IOP. In theory this would prevent hypotony (IOP <5mmHg) while allowing some reduction of IOP in the immediate post-operative period. There are two valved glaucoma drainage devices in common use today:
Krupin
Introduced in 1976,1 the first valved implant is still being manufactured today. Made of silicon and shaped like a disk, it has a surface area of 184mm2. The valve is a simple slit that is designed to open at an IOP of 11mmHg and close at 9mmHg. When it works as intended hypotony should not occur.
Ahmed™
Introduced by Marteen Ahmed™ in 1993,2 this polypropylene or silicon valved glaucoma drainage device is frequently used in the United States. Like the Krupin, it is designed to limit flow out of the eye under a given IOP. In the case of the Ahmed™ it has been engineered to open at an IOP of 8mmHg. It also has a similar surface area: 185mm2. A second plate can be attached to the first to add another 180mm2 of surface area for a total of 365mm2.
There is even a pediatric version with a surface area of 96mm2. Unlike the Krupin implant which is shaped like a disc, the Ahmed™ implant has a very unique shape. I think it looks a bit like a trilobite.
How to Choose?
With all the options available how do you (or your surgeon) choose the best glaucoma drainage device? Simply put, it’s a complex decision requiring the skill and nuanced understanding of glaucoma that only years of experience can provide. Some general guidelines, however, are as follows. Non-valved implants with large surface areas are preferred when the target IOP is in the low teens. Valved implants are often preferred if the IOP must be brought under control quickly or in those patients who are at greatest risk of complications from hypotony (such as those who are nearsighted).
References
1) Rollet M. Le drainage au irin de la chambre anterieure contre l’hypertonie et al douleur. Rev Gen Ophthalmol. 1906;25:481.
2) Zorab A. The reduction of tension in chronic glaucoma. Ophthalmoscope. 1912;10:258.
3) Stefansson J. An operation for glaucoma. Am J Ophthalmol. 1925;8:681-92.
4) Muldoon WE, Ripple PH, Wilder HC. Platinum implant in glaucoma surgery. Arch Ophthalmol. 1951;45:666.
5) Bock RH. Subconjunctival drainage of the anterior chamber by a glass seton. Am J Ophthalmol. 1950;33:
6) Koryllos K. The excision of the corneoscleral meshwork (trabeculectomy) as an antiglaucomatous operation. Delt Ell Ophthalol. 1967:35147–155.
7) Cairns JE. Trabeculectomy. Preliminary report of a new method. Am J Ophthalmol. 1968;66(4):673-679.
8) Molteno AC. New implant for drainage in glaucoma. Clinical tril. Br J Ophthalmol. 1969;53(9):606-615
Related Articles:
- Glaucoma Drainage Devices
- How Well Do Glaucoma Drainage Devices Work?
- Does It Matter Which Glaucoma Drainage Device Is Implanted?
- Risks Of Glaucoma Drainage Devices
- When Should Glaucoma Drainage Devices Be Considered?
- When Should Surgical Treatments Other Than Glaucoma Drainage Devices Be Considered?

David Richardson, MD
Medical Director, San Marino Eye
David Richardson, M.D. is recognized as one of the top cataract and glaucoma surgeons in the US and is among an elite group of glaucoma surgeons in the country performing the highly specialized canaloplasty procedure. Morever, Dr. Richardson is one of only a few surgeons in the greater Los Angeles area that performs Micropulse(r) P3 “Cyclophotocoagulation” (MP3) glaucoma laser surgery. Dr. Richardson graduated Magna Cum Laude from the University of Southern California and earned his Medical Degree from Harvard Medical School. He completed his ophthalmology residency at the LAC+USC Medical Center/ Doheny Eye Institute. Dr. David Richardson is also an Adjunct Assistant Professor of Clinical Ophthalmology at the Keck School of Medicine of USC. Twice weekly, he treats veterans at the VA Greater Los Angeles Veterans Healthcare System.