MIGS Where To Next?
A Roundtable Discussion Of Nondestructive Interventional Treatments For Open-angle Glaucoma
Dr. Ahmed: What percentage of the MIGS you perform is shunt/canal-based versus supraciliary or subconjunctival (Figure 1)?
Dr. Gallardo: I would say about 60 to 70% of my MIGS procedures are performed on the conventional outflow system. For me, it’s the safest system to work with because there’s virtually no risk for hypotony owing to the back wall of the episcleral venous system, and it has proven efficacy at reducing pressure and medication burden. After that, I prefer to use the suprachoroidal space, which has also been shown to be quite effective but has a tad bit higher risk for adverse events. Typically, my subconjunctival procedures are for patients who need a filter.
Dr. Richardson: I do about 90%-plus conventional outflow.
Dr. Singh: I’d say I perform about 70% natural outflow MIGS and 30% other devices. I still try and maximize natural outflow if possible as a first choice for many patients.
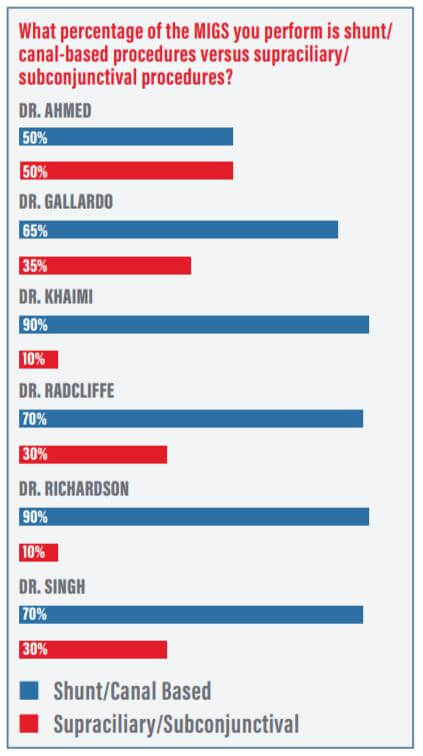
Dr. Radcliffe: I’ve got a severe glaucoma practice. If I set aside my tube shunts and just look at MIGS procedures, then I do at least 60% conventional outflow—maybe as high as 75%. The rest are split between supraciliary and subconjunctival MIGS. The fact that ABiC has insurance coverage available to my standalone patients and to a full spectrum of health insurance companies helps me provide this option to more people.
Dr. Khaimi: I’m a canal-based guy, so I’ve always focused on conventional outflow. I’ve dabbled with a lot of other things, too, but when I go subconjunctival, it’s usually to push that trabeculectomy farther downstream.
Dr. Ahmed: You guys have shared a lot of experience today. I thought we’d finish off the roundtable by talking about what excites you in the future of glaucoma therapy, whether it’s something about diagnostics, your practice, MIGS, or other treatments. What are you excited to see in the next 5 years?
I would say about 60 to 70% of my MIGS procedures are performed on the conventional outflow system. For me, it’s the safest system to work with because there’s virtually no risk for hypotony owing to the back wall of the episcleral venous system, and it has proven efficacy at reducing pressure and medication burden.
—Mark J. Gallardo, MD
Dr. Khaimi: Well, I wish we had MIGS a long time ago. I’m at a tertiary center, so I see many patients with severe glaucoma. We talk a lot about improving their quality of life with MIGS, but those options also dramatically improve the doctor’s quality of life. There’s a lot of less patient chair time. Patients are happy and doing well. It’s an extremely exciting time. We can now rejuvenate and restore the outflow system—it doesn’t get better than that. We were taught to bypass the natural outflow when we first started out, and now we can restore it.
Dr. Richardson: I love the idea that we’re getting to the point where we can talk about doing multiple less risky procedures together, or in short step-wise approach, to potentially achieve the same outcome as trabeculectomy or glaucoma drainage devices with far less risk. If we can get to the point where we do multiple MIGS procedures at the same time for moderate and severe glaucoma patients, then we’ll achieve the desired effect. For both patients and, as you mentioned, for the doctors as well, the low-risk outcomes are going to be wonderful.
Dr. Ahmed: Dr. Radcliffe, you always have something up your sleeve.
Dr. Radcliffe: Of course! Well, I look at myself 10 years ago using SLT only for severe glaucoma, and now it’s my primary therapy of choice. I’m hoping that in the next 10 years, or maybe even sooner, I can make that same kind of transition to bring standalone, canal-based procedures into my mindset and into my practice. In order to make this reflexive and be more proactive, we all have to change our attitudes and our thoughts. We need to get consensus. It takes time to adjust, but I think I’m there conceptually, and I look forward to turning that acknowledgement into practice.
It’s an extremely exciting time. We can now rejuvenate and restore the outflow system—it doesn’t get better than that. We were taught to bypass the natural outflow when we first started out, and now we can restore it.
—Mahmoud A. Khaim i, MD
Dr. Singh: I think it’s exciting that now we are pushing surgical intervention much earlier. Other technologies will help us. Diagnostic technologies will advance treatment, whether that’s blood flow, cerebrospinal fluid pressure, or even 24-hour pressure monitoring. With MIGS and other glaucoma treatment technologies, I think we have a better understanding from a diagnostic perspective. It’s funny, but MIGS procedures are making us realize how little we know about glaucoma. The more we learn, the more we have to do. For me, the exciting part is learning more about the pressure reduction that’s possible when we can couple new diagnostic modalities with early intervention.
MIGS procedures are making us realize how little we know about glaucoma. The more we learn, the more we have to do.
—Inder Paul Singh, MD
Dr. Ahmed: Well said. Dr. Gallardo, I’ll give you last word on this topic.
Dr. Gallardo: Just looking at the number of people in all the MIGS sessions here at ASCRS, we can see how these new procedures have excited everybody in ophthalmology. When I finished my fellowship, I attended a symposium about an older canaloplasty procedure. It was exciting at the time, because it was one of our first truly blebless procedures. We’ve come a long way from that to where we are today, using the same technology in a microinvasive nature. I’m hoping that at some point, we will have diagnostic imaging in our clinics that allows us to actually evaluate the outflow system and determine whether we should go with conventional outflow or a supraciliary approach. We may even be able to use the iTrack microcatheter to determine if the distal system is intact, or it might highlight a specific area that we can target with a stent procedure to maximize outflow. There is more to look forward to, and it’s kind of a cool time to be a glaucoma guy again.
References:
- Katz LJ, Steinmann WC, Kabir A, et al; SLT/Med Study Group. Selective laser trabeculoplasty versus medical therapy as initial treatment of glaucoma: a prospective, randomized trial. J Glaucoma. 2012;21(7):460-468.
- Cha ED, Xu J, Gong H. Variations in active areas of aqueous humor outflow through the trabecular outflow pathway. Presented at ARVO; May 3-7, 2015; Denver, Colorado.
- Grieshaber MC, Pienaar A, Olivier J, Stegmann R. Clinical evaluation of the aqueous outflow system in primary openangle glaucoma for canaloplasty. Invest Ophthalmol Vis Sci. 2010;51(3):1498-1504.
- Rhee, DJ. GFC Debate Re-Match 2018: Is Trabeculectomy Dead? ASCRS Glaucoma Subspecialty Day, April 13, 2018.
- Ellex iScience Inc. Data on File
A Roundtable Discussion Of Nondestructive Interventional Treatments For Open-angle Glaucoma
Cataract & Refractive Surgery Today
SUPPLEMENT | AUGUST 2018
Sponsored by Ellex Medical
Source: crstoday.com
Interventional Glaucoma: SLT and MIGS Series
Related Articles
- How To Choose Which Type Of Laser Trabeculoplasty To Have
- Laser Trabeculoplasty ALT, SLT, MLT for Glaucoma
- Selective Laser Trabeculoplasty for Glaucoma Just Got a Whole Lot Easier
- Will Selective Laser Trabeculoplasty (SLT) Result in a Need for New Spectacle Correction?
- Selective Laser Trabeculoplasty (SLT) After Incisional Glaucoma Surgery


